Updated August 20, 2024 by Authors Chris Cloney and Jon Barrett of Dust Safety Science
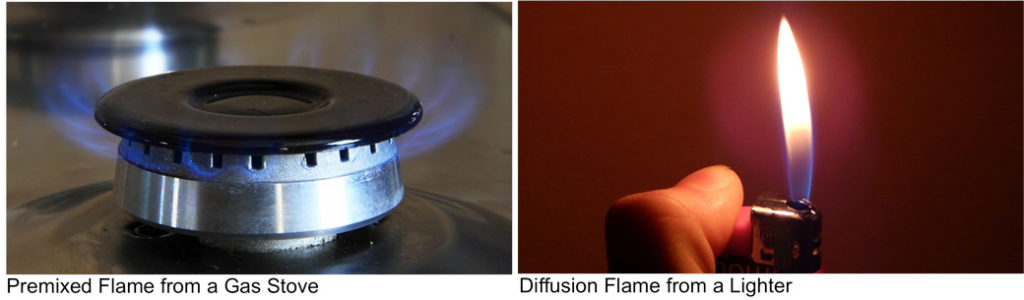
To understand the differences between flame propagating through a gaseous fuel and flame propagating through a combustible dust cloud, it is useful to compare the two basic categories of flame propagation.
Flame propagation refers to the propagation of the reaction zone or “combustion wave” through a combustible mixture. A laminar flame propagation also refers to the flame speed. Flame speed experiments are typically conducted over a range of equivalence ratios from lean to rich mixtures.
When the transport of heat and active species (free radicals) have initiated a chemical reaction in the adjacent layer of the combustible mixture, the layer itself becomes the source of heat and radicals and is then capable of initiating reaction in the next layer. A quantitative theory of flame propagation must be based on the transfer of heat and mass from the reaction zone to the unburned mixture. The enthalpy rise across the flame due to combustion is balanced by conduction from the reaction zone.
These two Flame Propagation categories are Premixed propagation and Non-Premixed propagation. A Gaseous flame will occur in both flame propagation categories, depending on how the fuel and oxidizer are mixed. As the mixing process is a prerequisite for having a dust explosion (e.g., see the dust explosion pentagon) a dust flame generally propagates in a non-premixed mode, although under certain conditions the flame can resemble a premixed one.
This article demonstrates the two basic categories of flame propagation for gas-only fuel systems and explores the important parameters and descriptions of each. The reader interested in a more in-depth coverage of this topic and with respect to the mathematical expressions that can be used in computer simulation models is encouraged to review Chapter 2 and Chapter 3 in the turbulent and multiphase combustion textbook by Kuo (Applications of Turbulent and Multiphase Combustion, Kuo, 2012).
A premixed flame results when the fuel and oxidizer are mixed prior to the passage of the reaction zone. An example premixed flame is shown in the photo above for a gas stove where natural gas (70-90% methane, 30%-10% other gases) and oxygen are mixed prior to being ignited inside the burner. The properties of a laminar premixed flame were explored in a previous post and the properties of turbulent premixed flames will be outlined in a future post. A laminar flame propagation refers to the flame speed at which an adiabatic, unstretched, premixed planar flame propagates relative to the unburned mixture. It is an important intrinsic property of a combustible mixture, containing information about diffusivity, reactivity, and exothermicity. Flame speed refers to the rapidness of the flame front motion from a reference point in a combustion process. It is a key parameter that can be influenced by the composition of the fuel mixture, with variations observed based on the concentrations of different gases like CO2 and CH4. According to Wikipedia, flame speed is the measured rate of expansion of the flame front in a combustion reaction. Whereas flame velocity is generally used for a fuel, a related term is explosive velocity, which is the same relationship measured for an explosive. Combustion engineers differentiate between the laminar flame speed and turbulent flame speed. Flame speed experiments are typically conducted over a range of equivalence ratios from lean to rich.
A non-premixed flame occurs when the fuel and oxidizer are not mixed prior to reacting. An example of this is the diffusion flame from a lighter as shown above. Lighter fuel is typically compressed butane which is liquid inside the lighter canister, but rapidly expands to gas once released from the lighter nozzle. The concentration of the butane near the nozzle is too high for combustion, and the flame cannot form until it mixes with the surrounding air. A Diffusion flame is typically much cooler than premixed flames as the mixing step limits the overall combustion.
A common way to characterize non-premixed flames is to look at the relative importance of the mixing and reaction processes. This type of comparison is frequently done in reactive systems by using dimensionless ratios. In the case of non-premixed flames, the Damkohler number ($latex \text{Da}$) is used to compare the timescale of mixing to the timescale of a chemical reaction.
$latex \text{Da} = \frac{t_{mix}}{t_{ch}}$
$latex t_{mix}: \text{[Time Scale for Mixing (s)]} $
$latex t_{ch}: \text{[Time Scale for Chemical Reaction (s)]} $
When the Damkohler number is very large ($latex \text{Da} \gg 1$), the reaction occurs in a very thin region between the fuel and oxidizer, and the flame speed is limited by the diffusion rate (e.g., the diffusion flame above). This is the basis for the Flame-Sheet model for diffusion flames (e.g., See Kuo (Applications of Turbulent and Multiphase Combustion, Kuo, 2012) Chapter 3.2). When the Damkohler number is very small ($latex \text{Da} \ll 1$), a premixed flame occurs as mixing is fast relative to the reaction. If the timescale of mixing and reaction are similar ($latex \text{Da} \approx 1$) a Partially-Premixed flame occurs where both the mixing and reaction processes are important to consider.
Flame Propagation and Safety
With respect to flame propagation, this process is a fascinating subject that has been extensively studied, with tests conducted, due to its significant implications in various fields, particularly in IC engines and fire safety. The propagation of a flame is influenced by numerous characteristics, including the energy release rate, the mixture of combustible species, and the conditions under which the combustible process occurs.
Flame propagation begins at a point where the energy from an ignition source initiates a combustible process. This process is then maintained as the flame spreads through the combustible mixture. The distance over which a flame can spread is determined by the mixture’s composition and the environmental conditions. For example, a richer fuel mixture generally results in a higher flame speed and greater propagation distance.
In combustion motors, understanding flame propagation is crucial for optimizing performance and efficiency. The duration of combustion and the means by which the flame spreads through the fuel-air mixture directly affect engine efficiency and emissions. Valid descriptions of these chemical reaction processes have been developed through extensive research, with the results providing valuable insights into improving engine designs.
Conclusion
With respect to a fire safety perspective, the study of flame propagation is essential for developing effective fire prevention and response strategies. The content and characteristics of materials involved in combustion, along with the conditions of their surroundings, determine how a fire will spread. Flame speed experiments are typically conducted over a range of equivalence ratios from lean to rich mixtures. The flame speed of dust can vary depending on a number of factors, including the type of dust, the particle size, and the air flow velocity. This information is required to establish safety protocols and security measures to protect human lives and property.
In conducting reviews of flame propagation, scientists have found that the connection between fuel and the combustible process is complex and multifaceted. Using this laminar flame propagation and flame speed knowledge including the range of equivalence ratios from lean to rich mixtures, must be respected, ensuring that research is conducted ethically, safely, and responsibly. Completing such studies on flame propagation allows for the development of improved safety measures and more efficient combustible applications, ultimately benefiting society as a whole.
