Published May 22, 2024 Authored by Dr. Chris Cloney and Jon Barrett of Dust Safety Science

Hazards of Magnesium Metal Dust and Magnesium Fires
Magnesium, a silvery-white metal, as a powder dust, presents a combustible hazard. Magnesium has an affinity for oxygen makes it highly reactive, resulting in intense exothermic reactions when exposed to heat or flame. In aerospace to automotive industries, magnesium powder poses a significant safety concern when handled in powder or dust form. Magnesium as a metal dust, is not the same as the mineral magnesium supplement or natural magnesium, magnesium citrate, citric acid, or a magnesium deficiency, affecting a persons blood pressure.
Combustible dust refers to fine particles suspended in the air, capable of igniting under certain conditions, leading to fires or explosions. When these particles accumulate in sufficient quantities and encounter an ignition source, the resulting conflagration can be devastating. Magnesium, when finely divided into dust, exhibits all the hallmarks of combustible materials, rendering it highly volatile in industrial settings. Magnesium combustible dust is generated in Metalworking shops, and fireworks manufacturing, from grinding, abrasive blasting, or cutting, which may cause dust clouds suspended in the air, causing a possible dust explosion hazard when suspended in the air. Although magnesium plays an important part in materials and production, process equipment and electrical equipment in these facilities add an additional ignition source for explosions.
These airborne magnesium powder particles can ignite with a spark or flame, leading to combustion and potentially causing a fire or explosion, forming magnesium oxide and hydrogen. Using dry sand to exclude the atmosphere oxygen from an explosion, is possible. Other materials and products, such as paper, wood, metal, coal, plastic, cotton, and rubber that may combust in powdered form are extensive and include a wide range of materials. Some materials, such as Zinc or Potatoes are not combustible in larger pieces but can be when pulverized to fine dust. The US Occupational Safety and Health Administration (OSHA) maintains a comprehensive list of combustible materials.
Standards and Safety for Combustible Magnesium Metal Dust
To address the unique challenges posed by combustible metals and metal dusts, the National Fire Protection Association (NFPA) developed NFPA 484, Standard for Combustible Metals. This comprehensive standard establishes guidelines and requirements for the safe handling, processing, and storage of combustible metals, including magnesium. By adhering to NFPA 484 guidelines, industrial facilities can ensure compliance with the most advanced fire and explosion safety protocols, minimizing the risk of magnesium-related incidents.
The NFPA 654 Standard for the Prevention of Fire and Dust Explosions from the Manufacturing, Processing, and Handling of Combustible Particulate Solids, provides guidance on combustible dust suppression and dust control. The NFPA 654 Standard is referenced by OSHA’s Combustible Dust National Emphasis Program (NEP) to identify dust hazards and define mitigation strategies that help protect life and property. The standard provides industry-recognized safety practices for facility and systems design, process equipment protection, fugitive dust control and housekeeping, ignition source identification and control, fire protection, training and procedures, inspection, and maintenance.
The Occupational Safety and Health Administration (OSHA) in the United States defines combustible dust as “a solid material composed of distinct particles or pieces, regardless of size, shape, or chemical composition, which presents a fire or deflagration hazard when suspended in air or some other oxidizing medium over a range of concentrations. Understanding these characteristics is vital for identifying combustible dust hazards, managing the risks associated with combustible dust, ensuring workplace occupational safety and compliance, and preventing a combustible dust explosion.
In addition, according to the International Magnesium Association, grinding dust should be captured in a wet dust collector system engineered for magnesium use only.
Magnesium Dust is Combustible and is an Explosion Hazard
Magnesium powder fires present many risks that demand meticulous attention and proactive mitigation strategies. The metal’s low ignition temperature, coupled with its rapid combustion rate, means that even a minor ignition source can trigger a catastrophic fire. Furthermore, magnesium fires emit intense heat and blinding light, posing a significant risk of thermal burns and eye injuries to personnel in proximity. On May 2, 2018 the Asia Times reported an Aluminum and Magnesium explosion in Changhua County, Taiwan. The dust explosion was reported to have occurred at a bike parts manufacturing facility. Aluminum and magnesium powder accumulated in a 10 square meter area in the facility and was ignited by a spark which caused the explosion.
Given the inherent hazards associated with magnesium powder as combustible dust, comprehensive preventive measures are paramount to safeguard personnel and property. Implementing robust dust collection and ventilation systems helps minimize the accumulation of magnesium dust, reducing the likelihood of ignition. Regular equipment maintenance and housekeeping practices further mitigate the risk of dust-related incidents. Determining the combustibility of dust present in your facility requires professional evaluation through laboratory testing. This process involves igniting a sample of the dust in a controlled environment and analyzing key parameters to assess the potential explosion risks. Magnesium fires and incidents have occurred all around the world.
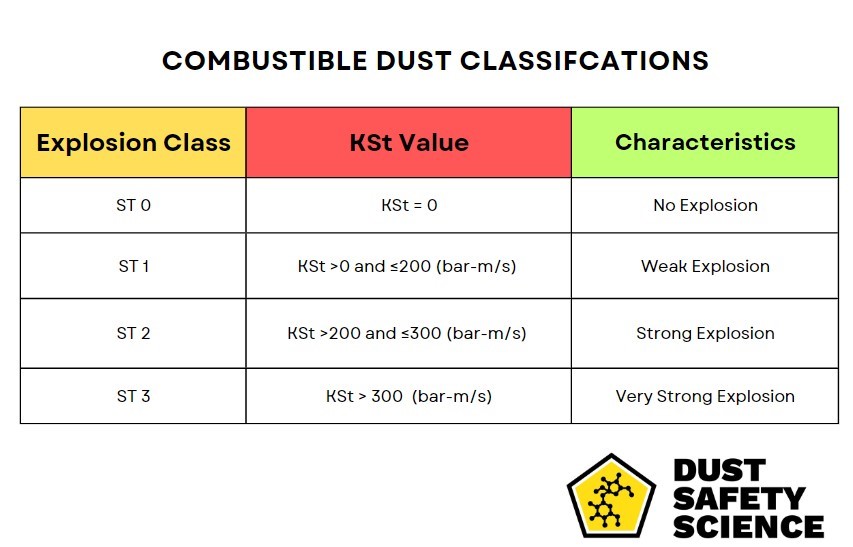
Magnesium Kst Value and Pmax Value, an Extremely Explosive Dust
Two critical elements of this analysis are the Kst value and the Pmax value. The Kst value measures the severity of an explosion that could occur if the dust ignites, with factors such as particle size, shape, and moisture content. Meanwhile, the Pmax value indicates the maximum pressure generated by a potential dust explosion. Magnesium powder dust has a KST value of 508 and a Pmax Value of 17.5, making magnesium powder dust extremely explosive.
Mitigating the Risks with Magnesium with Fires and Explosions
The risks associated with magnesium fires and explosions are multifaceted and demand meticulous attention. Magnesium fires are notoriously difficult to extinguish using conventional methods. Water, commonly employed to quench fires, exacerbates magnesium fires by releasing hydrogen gas upon contact, intensifying the blaze. Traditional dry chemical extinguishers also struggle to smother magnesium fires effectively, necessitating specialized firefighting techniques. Using dry sand to exclude the atmosphere oxygen from an explosion, is possible. Given the inherent hazards posed by magnesium fires, stringent preventive measures are imperative to safeguard both personnel and property. Employers must implement comprehensive dust control strategies, including regular cleaning of workspaces, ventilation systems, and machinery to minimize dust accumulation. Utilizing explosion-proof equipment and employing proper housekeeping practices can significantly mitigate the risk of ignition.
Furthermore, effective training programs are paramount to educate workers on the dangers of magnesium and instill best practices for safe handling and storage. Magnesium as a metal dust, is not the same as the mineral magnesium supplement, or natural magnesium, magnesium citrate, citric acid, vitamin d, affecting a person’s blood pressure and muscle function. Emphasizing the importance of personal protective equipment (PPE), such as flame-resistant clothing, goggles, and respirators, ensures that workers are adequately equipped to mitigate risks and respond swiftly in the event of an emergency. Despite robust preventive measures, the possibility of a magnesium fires and explosions cannot be entirely eliminated. Most people are not aware that to smother a potential fire using dry sand to exclude the atmosphere oxygen from an explosion. As such, organizations must develop comprehensive emergency response plans tailored to mitigate the specific challenges posed by magnesium fires. This includes establishing evacuation procedures, designating assembly points, and conducting regular drills to ensure swift and orderly responses in crisis situations.
Equally critical is the provision of specialized firefighting equipment designed specifically for combating magnesium fires. This may include dry sand and Class D, dry powder extinguishers containing agents such as graphite powder or sodium chloride, which effectively smother magnesium powder fires without exacerbating the situation. Ensuring the availability of these resources and training personnel in their use can significantly enhance emergency response capabilities.
About Dust Safety Science
For more from Dr. Chris Cloney on Magnesium Fires and Explosions, visit this Article: Magnesium Dust Fire and Explosions in Eaton Rapids, Michigan Injures Two and Cuts Automotive Parts Supply Line

Resources:
Visit Dust Safety Science (Global Incident Tracking)
Visit Dust Safety Academy (Resources, Training, and Events)
Visit Dust Safety Professionals (Need Help? Get Support Today!)
Visit Dust Safety Journal for the Complimentary, Dust Safety Science Monthly Journal
Subscribe to our Complimentary, Dust Safety Science Newsletter at Dust Safety Science Newsletter
Visit the Dust Safety Science blog for written articles on combustible dust safety including the latest research, expert opinions, and state-of-the-art in fire and explosion protection.
